FAMTER RANGES
THERAPEUTIC CLASSIFICATION: Antimalarial – Artemisinin-based combination therapy (ACT)




COMPOSITION:
FAMTER SUSPENSION (Artemether 240 mg / Lumefantrine 1440 mg)
FAMTER TABLET (Artemether 20 mg / Lumefantrine 120 mg)
FAMTER DS (Artemether 80 mg / Lumefantrine 480 mg)
INDICATIONS:
MAYDONTreatment of acute uncomplicated malaria caused by P. falciparum alone or with other plasmodium Spp in areas with significant drug resistance.
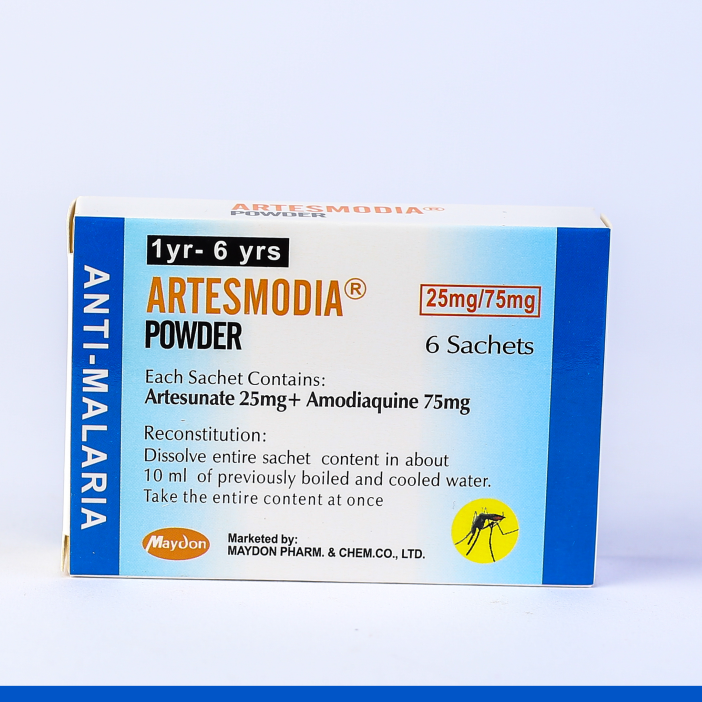
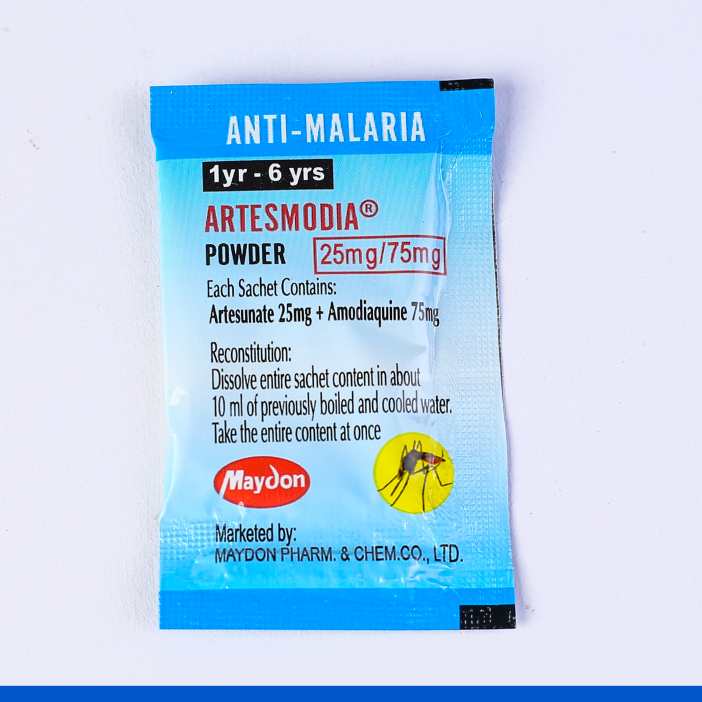
ARTESMODIA POWDER
THERAPEUTIC CLASSIFICATION: Antimalarial – Artemisinin-based combination therapy (ACT).
COMPOSITION:
Less than a year
- Artesunate 25 mg + Amodiaquine 75 mg
(3 Sachets)
1 - 6 years
- Artesunate 25 mg + Amodiaquine 75 mg
(6 sachets)
INDICATIONS:
MAYDONTreatment of acute uncomplicated malaria caused by P. falciparum (in children between the ages of 0 – 6 years) alone or with other plasmodium Spp in areas with significant drug resistance.

FANMET RANGES
THERAPEUTIC CLASSIFICATION: Antimalarial – Artemisinin-based combination therapy (ACT)


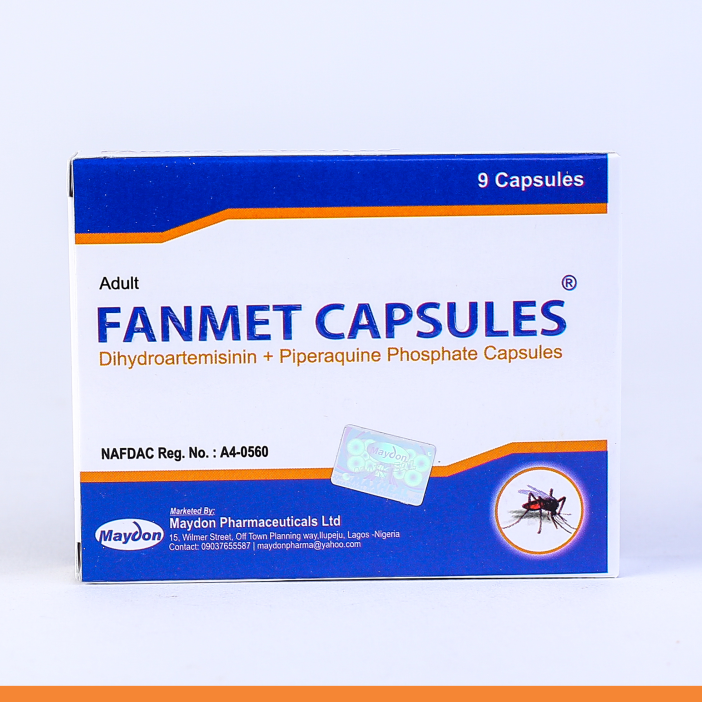
COMPOSITION:
FANMET SUSPENSION
- (Dihydroartemisinin 80 mg / Piperaquine 640 mg)
FANMET TABLET
- (Dihydroartemisinin 40 mg / Piperaquine 320 mg)
INDICATIONS:
MAYDONTreatment of P. falciparum and P. vivax malaria in adults and children.
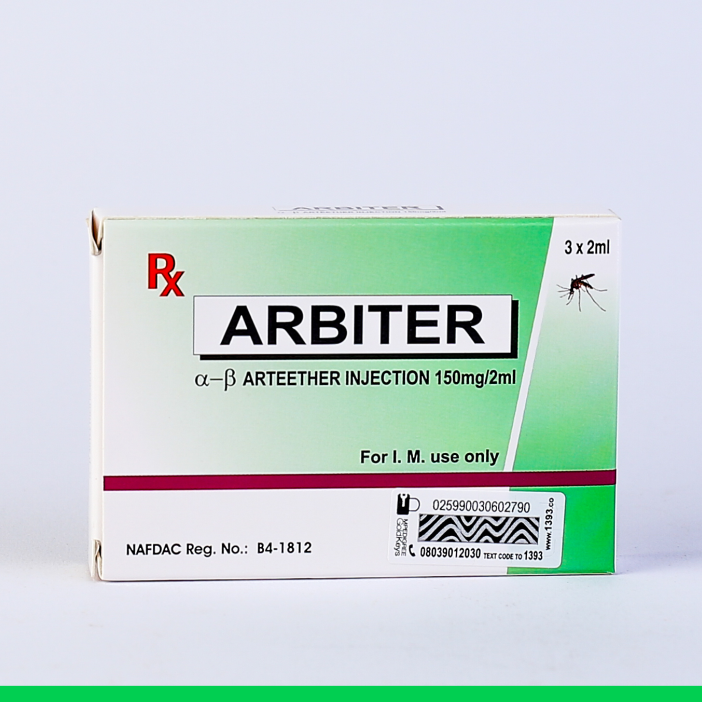
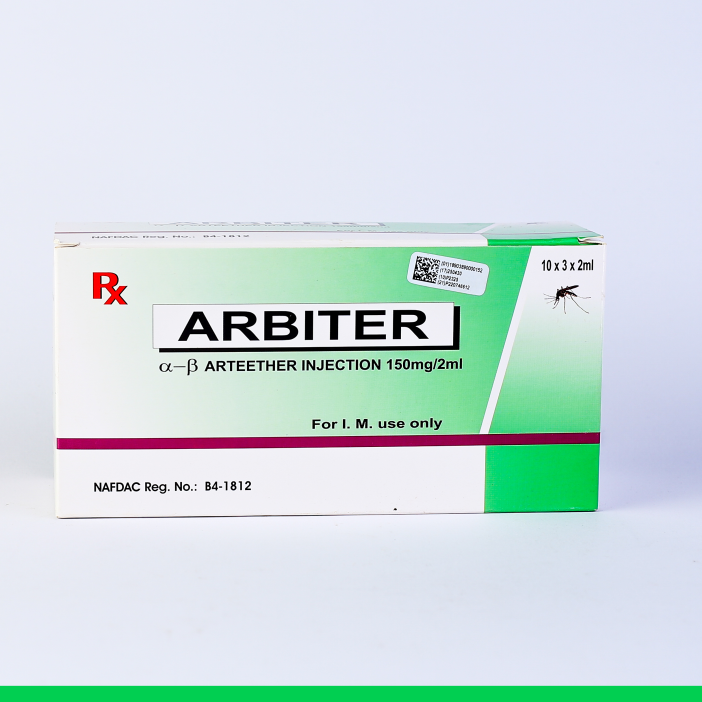
ARBITER INJECTION
THERAPEUTIC CLASSIFICATION: Antimalarial – Artemisinin synthetic derivative.
COMPOSITION:
α-β Arteether Injection 150 mg / 2 ml (for I.M. use only)
INDICATIONS:
MAYDONTreatment of severe P. falciparum malaria including cerebral malaria and as a second line treatment in Chloroquine resistant malaria. Not recommended to be used as a first line treatment of malaria.

ARTENITER INJECTION
THERAPEUTIC CLASSIFICATION: Antimalarial – Artemisinin derivative.

COMPOSITION:
Artemether Injection 80 mg / 1 ml (for I.M. use only)
INDICATIONS:
MAYDONTreatment of severe and complicated malaria both in adults and children in areas where there is multi-resistance.
MALAMOX
THERAPEUTIC CLASSIFICATION:
COMPOSITION:
INDICATIONS:
MAYDON